Abstract
Introduction
Lenalidomide-based regimens represent a cornerstone of treatment for newly-diagnosed transplant-ineligible patients with multiple myeloma (TI NDMM). Although lenalidomide-containing regimens are associated with improved outcomes, there is also significant toxicity leading to dose reductions and/or interruptions even among patients selected for clinical trial participation. Product monograph recommendations suggest full dose lenalidomide (25 mg) for CrCl ≥ 60 mL/min; however, there is limited data on how these dose modifications are applied in the real-world. Furthermore, it is unknown how real-world dosing strategies and modifications impact patient outcomes. Understanding the dosing, efficacy and tolerability of lenalidomide is important as this drug continues to form the backbone of many novel combination regimens.
The objective of our study was to understand the dosing, efficacy and tolerability of lenalidomide among TI NDMM patients started on lenalidomide-dexamethasone (Rd) in the real-world.
Methods
The Canadian Myeloma Research Group (CMRG) Database is a national multi-centre prospectively maintained disease-specific database. All consecutive TI NDMM patients treated with Rd outside of a clinical trial were analyzed up to 30/09/2020. Patients were stratified by starting lenalidomide dose. Within each dose category, age and renal function (eGFR <60 or ≥ 60 ml/min) were documented and dose modifications and treatment duration were characterized. Retrospective chart reviews were conducted to assess for causes of dose modification (all grade toxicity). To assess the long-term impact of dose reduction and minimize the effect of early mortality, a landmark analysis for progression-free survival (PFS) and overall survival (OS) 12 months following therapy initiation was conducted comparing those who received a dose reduction versus no reduction within the first year using the log-rank test.
Results
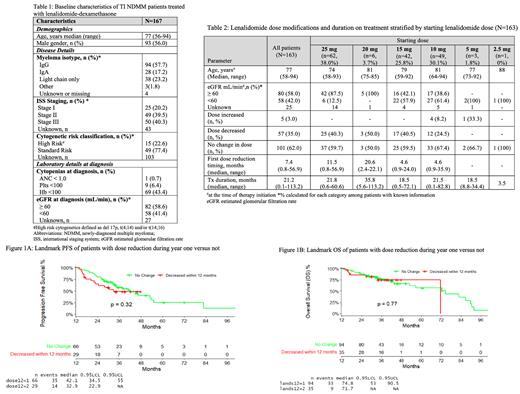
Of 298 TI NDMM patients receiving Rd in first-line treatment, 131 patients were excluded as they received the regimen on a clinical trial. Baseline characteristics of the 167 included patients are shown in Table 1. The median age was 77 with 22.6% having high-risk cytogenetics. Impaired renal function (eGFR < 60 ml/min) was present in 41.4% of patients. Median time from diagnosis to Rd initiation was 0.7 months (range 0.1 - 92.5).
Starting dose, age and renal function at therapy initiation, dose modification details and treatment duration are outlined in Table 2 for 163 patients. Sixty-two (38.0%) were started on a dose of 25 mg. The median age in this dose group was 74 years and 87.5% had normal renal function. One hundred and one patients (62.0%) were started on a dose < 25 mg of lenalidomide. Among this group 42.2% had normal renal function.
In the overall cohort, fifty-seven (35.0%) patients required dose reduction during therapy. Among patients started on 25 mg, 40.3% required dose reduction with the median time to first dose reduction of 11.5 months (range 0.8-56.9). Among patients started on < 25 mg, 31.7% required dose reduction with details shown in Table 2. Dose reduction reasons were documented in 35.0% patients. Most frequent causes of dose reduction (>20% of the patients) included fatigue (31.6%), neutropenia (29.8%), diarrhea (22.8%), rash (22.8%) and thrombocytopenia (21.1%).
For the entire cohort, the median follow-up was 33.2 months (95% Cl 0.5-113.2). The ORR was 78.7% with 52.0% ≥ VGPR. The median PFS and OS was 21.0 months (95% CI 14.9-27.4) and 55.5 months (95% CI 45.7-84.0) respectively. In a landmark analysis at 12 months following therapy initiation, there were no significant differences noted for PFS and OS between patient groups that required dose modification versus not in the first year following diagnosis (Figure 1A and B).
Conclusion
In this real-world observational study, lenalidomide dose modifications were common both at diagnosis and during therapy. Among patients started on <25 mg, 42% had normal renal function suggesting that additional factors affected clinician dosing strategy. Furthermore, although 35% of the cohort required dose reduction during therapy, dose reductions in the first year did not have a significant impact on PFS and OS. Our study highlights that although dose modifications are common in the real-world, lenalidomide-based regimens continue to be effective among TI NDMM patients.
Mian: Celgene, Janssen, Amgen, Takeda, Sanofi, GSK: Honoraria; Janssen: Research Funding. Leblanc: Janssen Canada: Membership on an entity's Board of Directors or advisory committees; BMS/Celgene Canada: Membership on an entity's Board of Directors or advisory committees; Amgen Canada: Membership on an entity's Board of Directors or advisory committees; Sanofi Canada: Membership on an entity's Board of Directors or advisory committees; Takeda Canada: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Research Funding. Louzada: Celgene: Honoraria; Janssen: Honoraria; Pfizer: Honoraria; Amgen: Honoraria. McCurdy: Sanofi: Honoraria; GSK: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Venner: Celgene: Research Funding; Takeda: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Amgen: Research Funding. Jimenez-Zepeda: BMS, Amgen, Takeda, Janssen: Honoraria. Sebag: Karyopharm Therapeutics: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Bristol Myers-Squibb: Consultancy, Honoraria; Janssen: Research Funding. White: Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Antengene: Consultancy, Honoraria; Forus: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Karyopharm Therapeutics Inc.: Consultancy, Honoraria. Song: Celgene: Research Funding; Celgene, Janssen, Amgen, Takeda: Honoraria. Reiman: Compositions and Methods for Inhibiting Blood Cancer Cell Growth. Canadian Patent Pending 176000 (2017-10-20) Peptides for the Treatment of Resorptive Bone Disease. Murugesan A and Reiman T. United States Provisional Patent; 62/249,471 (2015-11-02). Cance: Patents & Royalties; Myeloma Canada, Canadian Institutes of Health Research, New Brunswick Health Research Foundation, Canadian Cancer Society, Terry Fox Research Institute, AstraZeneca, Roche, Pfizer, Amgen, BristolMyersSquibb,: Research Funding; Myeloma Canada: Membership on an entity's Board of Directors or advisory committees. Kotb: Amgen: Honoraria; Sanofi: Honoraria, Research Funding; Merck: Honoraria, Research Funding; Akcea: Honoraria; Pfizer: Honoraria; Karyopharm: Current holder of individual stocks in a privately-held company; Celgene: Honoraria; BMS: Honoraria; Takeda: Honoraria; Janssen: Honoraria. Reece: Karyopharm: Consultancy, Research Funding; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Millennium: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Sanofi: Honoraria; BMS: Honoraria, Research Funding; GSK: Honoraria; Janssen: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal